What is the function of diffusion material?
Diffusion is the physical process through which a substance moves from a region of higher concentration to a region of lower concentration, resulting in a consistent emission of a gas or liquid over time via a diffusion material. The most common example seen is at-home fragrance delivery products, which release and diffuse a scent into the air. In addition to that, though, it also serves an important function in life science products such as bioprocessing spargers, electronics applications such as diffusion tubes for semiconductor processing, as well as commercial applications such as aquarium bubblers.
Porex has a long history of designing and manufacturing porous plastic and porous fiber solutions for various sparging and diffusion applications. Utilizing our material science and design expertise, Porex products are designed so that the diffusion media—comprised of omnidirectional matrices—yield a consistent, evenly distributed vapor release. Our products are custom-engineered for manufacturers to address applicational constraints and meet specific end-user performance requirements.


Talk with a Material Science Expert
Webinar
In this webinar preview, explore diffusion material as we discuss:
- How diffusion works
- Why diffusion requires vaporization and volatility
- The typical problems that are solved by diffusion
You’ll learn about the four main markets that benefit from diffusion, the types of porous polymers needed for successful diffusion, and so much more.
How diffusion material works with porous polymers
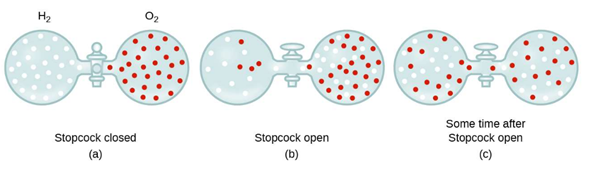
Diffusion occurs when molecules move from an area where they are common (high concentration) to an area where they are scarce (low concentration). This continues over time until molecules become evenly spread out, as shown in the figure below.
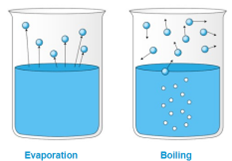
For many diffusion applications, vaporization must occur to facilitate the intended function of the device. Vaporization refers to an element’s phase transition from a liquid to a gas (vapor) phase. This can be accomplished, typically, via one of two methodologies – evaporation or boiling. Evaporation occurs on the surface of a liquid at temperatures below the boiling point at a given pressure. Boiling occurs when the equilibrium vapor pressure of the substance is greater than the atmospheric pressure. By adding heat to a liquid, it, in turn, begins to absorb that heat, raising its own temperature until reaching its boiling point. It then stops absorbing heat and begins to vaporize. Depending on the liquid material, it may have high or low volatility. A volatile liquid is described as a liquid that vaporizes readily under atmospheric conditions. This volatility is important to consider when designing your diffusion solution, along with temperature, humidity, and useful life.
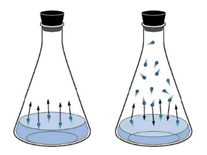
Design challenges solved by diffusion material
- Getting consistent release of an air freshener scent or insecticide into a room
- Delivering an even spread of oxygen or other process gas into a system
- Ensuring an even dispersion of light onto a surface or into a focus area
- Getting a liquid, gas, or particles moved into a uniform distribution to aid in a process

Related Technologies

Sintered Porous Plastics
Sintered porous plastics are created using a combination of heat and pressure to bond the materials together.

Porous Membranes
Porous fibers consist of bonded fibrous strands which create two-dimensional cross-sections that can be extruded to create three-dimensional shapes.
Register for our On-Demand Webinar
Using Porous Plastics for Diffusion